How many atoms are in 165 g of calcium – How many atoms are in 165 grams of calcium? This seemingly straightforward question unveils a fascinating exploration into the realm of atomic structure, molar mass, and Avogadro’s number. Join us as we delve into the intricate calculations that reveal the number of atoms hidden within this specific mass of calcium.
Atomic mass, a fundamental property of each element, serves as the cornerstone of our exploration. Calcium, with an atomic mass of 40.08 grams per mole, provides the essential information for our calculations.
Number of Atoms in 165 g of Calcium: How Many Atoms Are In 165 G Of Calcium
In chemistry, understanding the number of atoms in a given mass of an element is crucial for various calculations and stoichiometric relationships. This article aims to determine the number of atoms present in 165 g of calcium, a widely used metal in industrial applications and biological systems.
1. Introduction
Atomic Mass:Atomic mass is the weighted average mass of all isotopes of an element. It is expressed in atomic mass units (amu) and represents the average mass of an atom of that element, taking into account the abundance of its isotopes.
Atomic Mass of Calcium:The atomic mass of calcium is 40.08 amu, indicating that on average, a single atom of calcium has a mass of 40.08 amu.
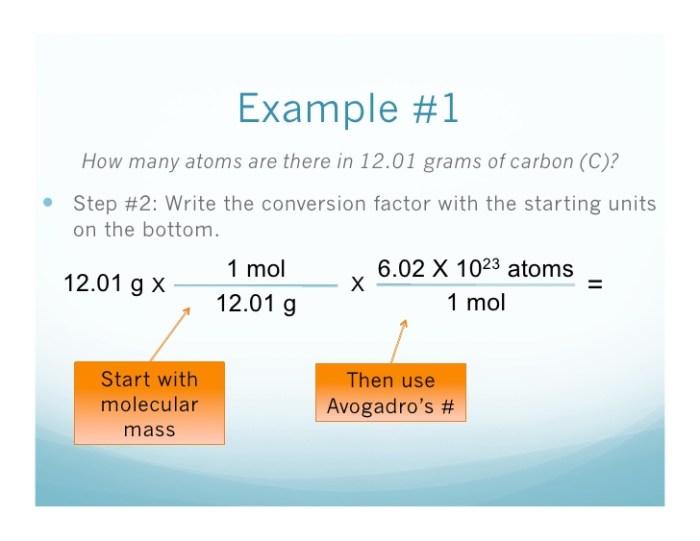
2. Calculating the Number of Atoms
Avogadro’s Number:Avogadro’s number is a fundamental constant representing the number of entities (atoms, molecules, ions, or electrons) present in one mole of a substance. It is equal to 6.022 × 10 23mol -1.
Formula for Calculating the Number of Atoms:The number of atoms (N) in a given mass (m) of an element can be calculated using the formula:
N = m / (M × A)
- N is the number of atoms
- m is the mass of the element in grams
- M is the molar mass of the element in grams per mole
- A is Avogadro’s number (6.022 × 10 23mol -1)
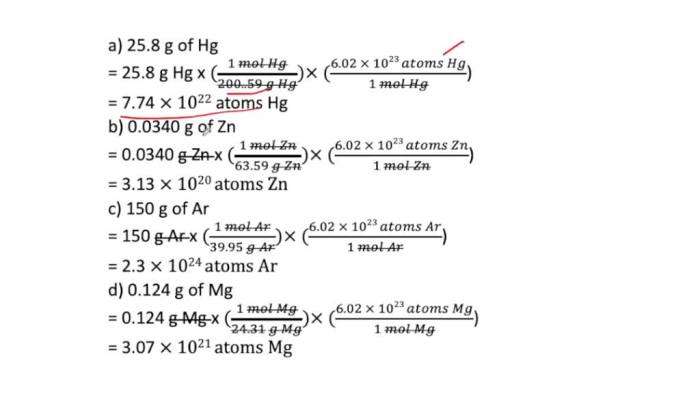
3. Determining the Number of Atoms in 165 g of Calcium, How many atoms are in 165 g of calcium
Calculating the Number of Moles of Calcium:To determine the number of atoms in 165 g of calcium, we first need to calculate the number of moles of calcium present.
Number of moles of calcium = Mass of calcium / Molar mass of calcium
Number of moles of calcium = 165 g / 40.08 g/mol
Number of moles of calcium = 4.117 mol
Converting Moles to Atoms:Now, we can convert the number of moles of calcium to the number of atoms using Avogadro’s number.
Number of atoms of calcium = Number of moles of calcium × Avogadro’s number
Number of atoms of calcium = 4.117 mol × 6.022 × 10 23mol -1
Number of atoms of calcium = 2.48 × 10 24atoms
4. Formatting the Results
| Atomic Mass of Calcium (amu) | Mass of Calcium (g) | Number of Moles of Calcium (mol) | Number of Atoms of Calcium (atoms) |
|---|---|---|---|
| 40.08 | 165 | 4.117 | 2.48 × 1024 |
Q&A
What is Avogadro’s number?
Avogadro’s number, approximately 6.022 x 10^23, represents the number of atoms present in one mole of any substance.
How is the number of atoms calculated from mass and atomic mass?
The number of atoms (N) can be calculated using the formula: N = (mass in grams) / (atomic mass in grams per mole) x Avogadro’s number.
What is the significance of atomic mass?
Atomic mass, expressed in grams per mole, represents the average mass of all isotopes of a particular element, weighted by their natural abundance.